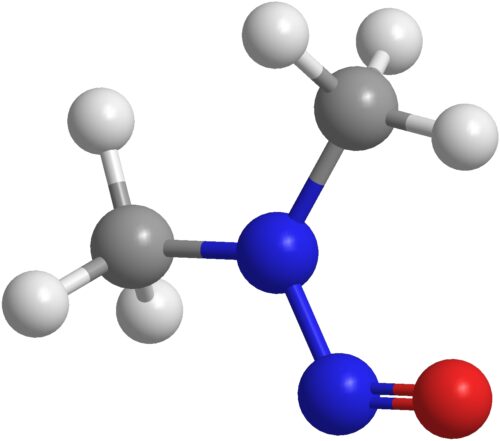
NDMA
The linkage between nitrosamines and cancer was first postulated by William Lijinsky in 1970. Then, in 2018, N-nitroso-dimethylamine (NDMA)) was detected in an active pharmaceutical ingredient, Valsartan (an Angiotensin-II-receptor antagonist). Finally, the FDA issued a guidance for the industry, “Control of Nitrosamine Impurities in Human Drugs”, in the fall of 2020. However, the guidelines continue to evolve. There has been an update in March of 2021, with ongoing risk assessments. Other regulatory agencies have instituted their own, along with updates. For example, since our blog series on nitrosamines, there have been some regulatory updates from the European Medicines Agency (EMA). Their new guidelines are outlined in a document entitled, “Questions and Answers for Marketing Authorization Holders/Applicants on the CHMP Opinion for the Article 5(3) of Regulation (EC) No 726/2004 Referral on Nitrosamine Impurities in Human Medicinal Products.”1 The updated section answers the crucial question, “Which limits apply for nitrosamines in medicinal products?”
The answers are provided with a definition of nitrosamines and acceptable exposures:
“The ICH M7(R1) guideline defines N-nitrosamines as substances of the “cohort of concern” for which limits in medicinal products refer to the so-called substance-specific acceptable intake (AI) (the Threshold of Toxicological Concern, TTC, value of 1.5 ug/day cannot be routinely applied) which is associated with a negligible risk (theoretical excess cancer risk of <1 in 100,000 over a lifetime of exposure). The calculation of AI assumes a lifelong daily administration of the maximum daily dose of the medicinal product and is based on the approach outlined in the ICH M7(R1) guideline as well as the principles described in relation to the toxicological evaluation in the assessment report of the CHMP’s Article 5(3) opinion on nitrosamine impurities in human medicinal products.”1,2 (A previous blog examined TTC, here.)
A Useful List of Nitrosamine Limits
In Appendix 1 found on the EMA site, there is a list of more than eighty nitrosamines listed, along with CAS numbers, known medicinal sources, CPCA (Carcinogenic Potency Categorization Approach) categories, and their guidance publication dates.3,4
Some Caveats
There are some exceptions that should be considered. For example, the EMA states, “The ‘less than lifetime’ (LTL) approach should not be applied in calculating the limits as described above but can only be considered after consultation with competent authorities as a temporary measure until further measures can be implemented to reduce the contaminant at or below the limits defined above.”1
Additionally, those medications intended for advanced cancers also have some exceptions. For example, “If the active substance itself is mutagenic or clastogenic at therapeutic concentrations, N-nitrosamine impurities should be controlled at limits for non-mutagenic impurities according to ICH M7(R1).”
There is also guidance when one or more than one nitrosamines may be present. For the latter, the guidance advises one of two approaches:
- The total daily intake of all identified N-nitrosamines is not to exceed the AI of the most potent N-nitrosamine identified.
- The total risk level calculated for all identified N-nitrosamines is not to exceed 1 in 100,000. The approach chosen needs to be duly justified by the MAH (Marketing Authorization Holder)/Applicant.1
Final Thoughts on Nitrosamines
Nitrosamine guidance worldwide is ever-evolving, yet the impetus to quantify and regulate them is clear. There will doubtlessly be further updates to regulations. AMPAC Analytical Laboratories – an SK pharmteco company (AAL) is an industry leader in the detection of nitrosamines and other genotoxic impurities (GTI), We have the specialized expertise, equipment, and methodologies to detect these impurities by gas chromatography or high-performance liquid chromatography coupled with mass spectrometry to support your API project. Also, importantly, AAL can assist in navigating those projects within today’s regulatory landscape. Please contact us with any specific questions or to receive a quote for nitrosamines or other GTIs.
References
- https://www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-questions-answers-marketing-authorisation-holders/applicants-chmp-opinion-article-53-regulation-ec-no-726/2004-referral-nitrosamine-impurities-human-medicinal-products_en.pdf
- ICH M7 Principles – Impurity Identification and Control (europa.eu)
- https://www.ema.europa.eu/en/human-regulatory/post-authorisation/referral-procedures/nitrosamine-impurities
- Appendix 1 Nitrosamine AIs (europa.eu)
Resources
AMPAC Analytical
- Watch Our On-Demand Webinar on Genotoxic Impurities
https://ampacanalytical.com/events/on-demand-webinar/ - AMPAC Analytical’s Nitrosamine Impurities Testing Webpage
https://ampacanalytical.com/laboratory-services/ampac-analytical-offers-ndma-analyses-of-api-and-dp/#1644962917933-ed2cb310-b026
General Information on Nitrosamines
- An Organic Chemist’s Guide to NNitrosamines: Their Structure, Reactivity, and Role as Contaminants
https://pubs.acs.org/doi/10.1021/acs.joc.0c02774?ref=pdf
Nitrosamine and Pharmaceuticals
- Information about Nitrosamine Impurities in Medications
https://www.fda.gov/drugs/drug-safety-and-availability/information-about-nitrosamine-impurities-medications - Prevalence of Nitrosamine Contaminants in Drug Samples: Has the Crisis Been Overcome?
https://doi.org/10.1002/ardp.202200484 - Nitrosamines Impurities in Pharmaceuticals, the Abrupt Challenges They Bring, and Approaches to Tackle the Risk
https://doi.org/10.1016/j.xphs.2022.07.016
- Critical Analysis of Drug Product Recalls Due to Nitrosamine Impurities
https://doi.org/10.1021/acs.jmedchem.0c02120 - Estimated Cancer Risks Associated with Nitrosamine Contamination in Commonly Used Medications
https://doi.org/10.3390/ijerph18189465
Regulatory Experiences with Root Causes and Risk Factors for Nitrosamine Impurities in Pharmaceuticals
https://doi.org/10.1016/j.xphs.2022.12.022




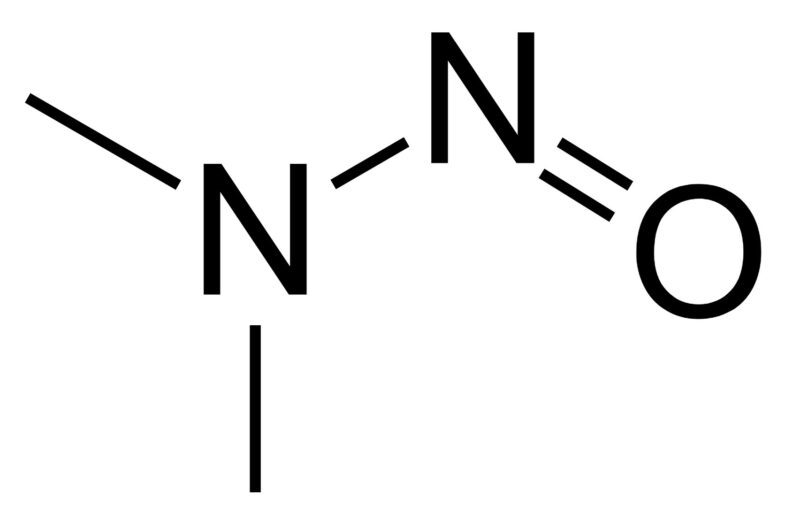

 Elemental impurities in food have been in the news recently, with reports of everything from lead being found in baby food to arsenic, cadmium, and assorted heavy metals in dark chocolate and other foods.
Elemental impurities in food have been in the news recently, with reports of everything from lead being found in baby food to arsenic, cadmium, and assorted heavy metals in dark chocolate and other foods.
