The Background and Concerns of PFAS
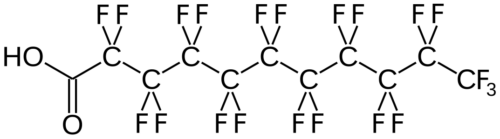 (Per- and) PolyFluoroAlkyl Substances (PFAS) are a class of ubiquitous chemicals that have been found in water, air, fish, and soil across the nation and worldwide. Known as “Forever Chemicals,” there are thousands of different PFAS, and they are present in consumer, commercial, and industrial products.1 Having one of the strongest bonds in organic chemistry, their structures proved to be resistant to heat, water, oil, and degradation.2 They are found in “food packaging and non-stick cookware, cosmetics, waterproof and stain-proof textiles and carpet, aqueous film forming foam (AFFF) to fight Class B fires, and as part of metal plating processes.”3 Teflon and Scotchgard were two of the pioneering products to utilize these fluoropolymers. The two most common PFAS are perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS).
(Per- and) PolyFluoroAlkyl Substances (PFAS) are a class of ubiquitous chemicals that have been found in water, air, fish, and soil across the nation and worldwide. Known as “Forever Chemicals,” there are thousands of different PFAS, and they are present in consumer, commercial, and industrial products.1 Having one of the strongest bonds in organic chemistry, their structures proved to be resistant to heat, water, oil, and degradation.2 They are found in “food packaging and non-stick cookware, cosmetics, waterproof and stain-proof textiles and carpet, aqueous film forming foam (AFFF) to fight Class B fires, and as part of metal plating processes.”3 Teflon and Scotchgard were two of the pioneering products to utilize these fluoropolymers. The two most common PFAS are perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS).
Health Concerns of PFAS
Some of the most frequently cited health concerns associated with PFAS include adverse cardiovascular, immunity, developmental, and hepatic effects.3,4The most commonly heard refrain to minimize these concerns is that if they are so prevalent, why are there
not more health issues associated with them? In fact, “The Lancet Commission on Pollution and Health reported that pollution was responsible for 9 million premature deaths in 2015, making it the world’s largest environmental risk factor for disease and
premature death.” This was updated in 2019, and those numbers held steady, accounting for one in six deaths worldwide.5/i> While this number includes all types of pollution, the impacts are clear.
The Exposure Concerns of PFAS Are Regulatory and Legal
Due to their combination of persistence, pervasiveness, mobility, and the ability of some to bioaccumulate (or build up in animals and humans), they have been in the news recently too.6 Predictably, they are also now moving through the courts.7-9 Some of the most common areas of litigation are directed at PFAS found in drinking water and firefighting foam. The regulatory initiatives are also increasing. These address a range from water and soil to numerous manmade products including food packaging.10,11 The European Chemicals Agency (ECHA) and the NIH have a wealth of guidance and regulations that apply to PFAS.12,13
PFAS Detection
The EPA has useful direction for analytical methods development and sampling research that outlines the “laboratory validation process following a particular rulemaking or guidance effort and are available to support regulatory or guidance activities.”14 For
technique and equipment, PFAS are typically analyzed by mass spectrometry, coupled with gas chromatography or liquid chromatography (GCMS and LCMS), which enables detection in the low parts per billion.
AMPAC Analytical has years of experience and numerous experts in trace analysis by mass spectrometry who can assist with method development for high-volume analyses for both common and atypical sample matrices that will allow you to stay ahead of evolving regulatory concerns. Please contact us with specific questions or to receive a quote for PFAS quantitation.
References
- PFAS Explained | US EPA
- Understanding Organofluorine Chemistry. An Introduction to the C–F bond – Chemical Society Reviews (RSC Publishing)
- PFAS Health Effects Database: Protocol for a Systematic Evidence Map – ScienceDirect
- Toxicological Profile for Perfluoroalkyls (cdc.gov)
- Pollution and Health: a Progress Update – The Lancet Planetary Health
- ‘Forever Chemicals’ Are Everywhere. What Are They Doing to Us? – The New York Times (nytimes.com)
- PFAS Settlements: Future of PFAS Litigation Landscape to be Determined by Upcoming Decision | Reuters
- PFAS: The New Frontier of Product Liability – ProQuest
- DuPont, Corteva, and Chemours Announce Resolution of Legacy PFAS Claims | DuPont
- Trends in the Regulation of Per- and Polyfluoroalkyl Substances (PFAS): A Scoping Review
- PFAS in Food Packaging: State-by-State Regulations – September 2023 | Bryan Cave Leighton Paisner – JDSupra
- Per- and Polyfluoroalkyl Substances (PFAS) – ECHA (europa.eu)
- Guidance on PFAS Exposure, Testing, and Clinical Follow-Up – NCBI Bookshelf (nih.gov)
- PFAS Analytical Methods Development and Sampling Research | US EPA
Resources
- General Information and Guidance
o Per- and Polyfluoroalkyl Substances (PFAS) | US EPA
o What Products Contain PFAS & How to Protect Yourself (consumernotice.org)
o PFAS Explained | US EPA
o Perfluoroalkyls | Toxicological Profile | ATSDR (cdc.gov)
o Key EPA Actions to Address PFAS | US EPA - Health Concerns
o Exposures to Per-and Polyfluoroalkyl Substances (PFAS): Potential Risks to Reproductive and Children’s Health – ScienceDirect - Regulations
o PFAS Regulations: Past and Present and Their Impact on Fluoropolymers | Perfluoroalkyl Substances | Books Gateway | Royal Society of Chemistry (rsc.org)



 Elemental impurities in food have been in the news recently, with reports of everything from lead being found in baby food to arsenic, cadmium, and assorted heavy metals in dark chocolate and other foods.
Elemental impurities in food have been in the news recently, with reports of everything from lead being found in baby food to arsenic, cadmium, and assorted heavy metals in dark chocolate and other foods.