AMPAC Analytical (AAL) is a global leader that supports all analytical requirements for pharmaceutical intermediates, Active Pharmaceutical Ingredients (APIs), and formulated drug products. From Analytical Method Development and Implementation to Analytical Method Validation and stability, AMPAC Analytical is fully equipped with fully compliant cGMP instrumentation geared toward product analysis and product release. AAL supports spectroscopy, chromatography, particle size distribution, calorimetry, osmolality, mass spectrometry, and ICH Stability project requirements.
Analysis Blog
In the entries below, we’ll share our thoughts and comments on these areas and other topics of interest. Please feel free to contact us to suggest topics of interest.
You can also sign up for the Analysis Newsletter, which includes blogs and other content directly to your mailbox. It is delivered approximately every two weeks, and you can subscribe here.
Newsletter Signup Form
A Survey of Material Science and Physical Characterization Techniques and Equipment for Small Molecules – Part I
Material science and physical characterization are crucial to ensure drug products’ or drug substances’ identities with measurements and analysis. There are many types of techniques and equipment to do this, each with its own features and qualities. In this blog, the first part of a series, we will review the advantages of a range of … Continue reading A Survey of Material Science and Physical Characterization Techniques and Equipment for Small Molecules – Part I »
Setting the Standard with Reference Standard Qualifications
The term Reference Standard Qualifications (RSQ) “is defined as a list of tests, references to analytical procedures, and appropriate acceptance criteria,”1 and incorporate these quantitative reference standards to do so: purity, potency, identification, impurities content, and generally full characterization. Identity Testing There are a range of tests to ensure the identity of the drug product … Continue reading Setting the Standard with Reference Standard Qualifications »
Shedding Light on Photo-Stability Forced Degradation
Forced Degradation is the testing of a drug product or drug substance using situations more taxing than those if conditions were simply accelerated. This type of stability testing is designed to demonstrate various degradation pathways within the product or substance and assists with the development of the product itself, along with its packaging. Within forced degradation … Continue reading Shedding Light on Photo-Stability Forced Degradation »
Dissolution Testing and Development
An Introduction to Dissolution Testing and Development Dissolution testing is the monitoring of drug substances in a controlled environment from a solid dosage form (i.e., capsules, tablets) to a solution state. These “tests to characterize the dissolution behavior of the dosage form, …also take disintegration characteristics into consideration, are usually conducted using methods and apparatus … Continue reading Dissolution Testing and Development »
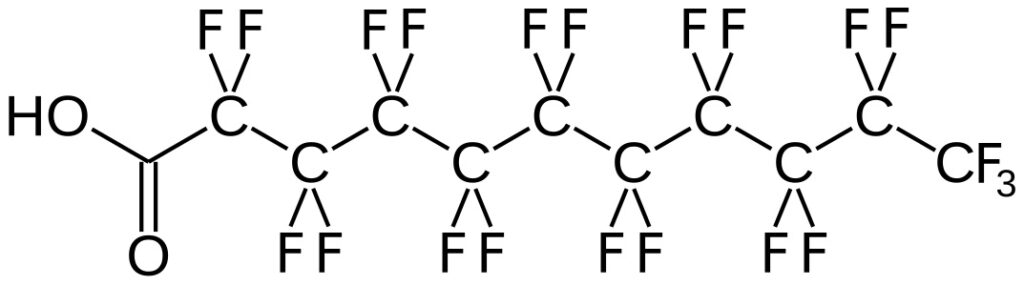
Some Background and Concerns About PFAS
The Background and Concerns of PFAS (Per- and) PolyFluoroAlkyl Substances (PFAS) are a class of ubiquitous chemicals that have been found in water, air, fish, and soil across the nation and worldwide. Known as “Forever Chemicals,” there are thousands of different PFAS, and they are present in consumer, commercial, and industrial products.1 Having one of … Continue reading Some Background and Concerns About PFAS »
Gas Chromatography – An Introduction
A Brief History of Gas Chromatography Gas chromatography (GC) is one of the most important and prevalent analytical tools available to chemists. It was invented in 1952 by A.T. James and A.J.P. Martin as an outgrowth of research dating back to the previous decade.1-6 Their early techniques on adsorption and partition enabled some of the … Continue reading Gas Chromatography – An Introduction »
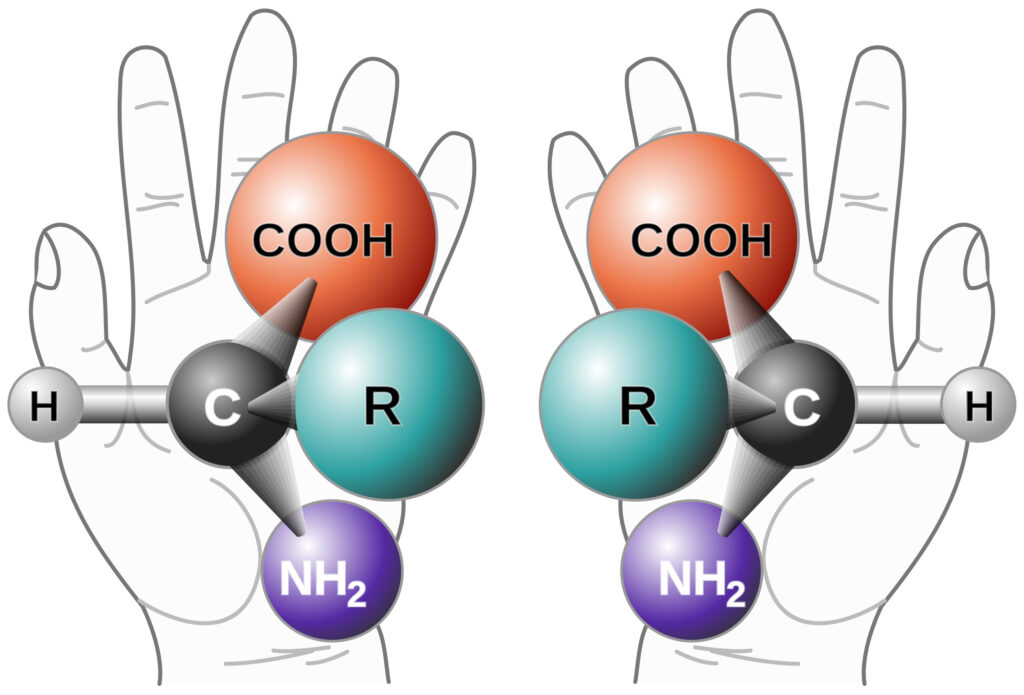
Chiral Purity Analysis – The Need to Know What Both Hands Are Doing
Background on Chiral Purity Chirality refers to the phenomenon that occurs when a mirror image cannot be superimposed. It is sometimes called “optical rotation”. The origin is from the late 19th-century Greek word kheir (‘hand’) and is one of the easiest demonstrations of the concept. Although a person’s hands may appear virtually identical, if they … Continue reading Chiral Purity Analysis – The Need to Know What Both Hands Are Doing »
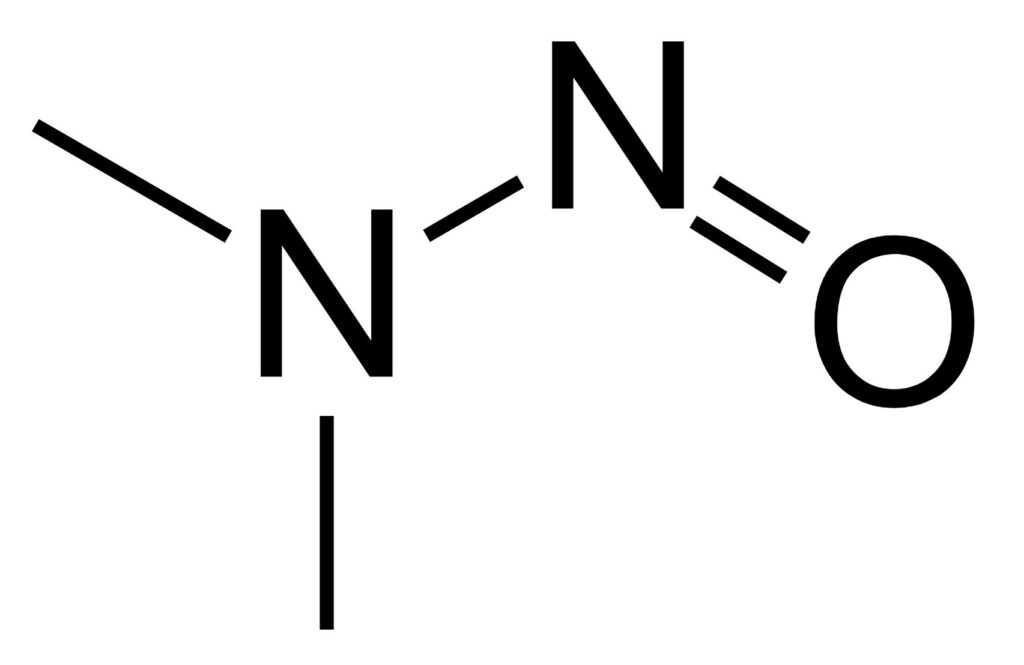
Nitrosamines – An Update
The linkage between nitrosamines and cancer was first postulated by William Lijinsky in 1970. Then, in 2018, N-nitroso-dimethylamine (NDMA)) was detected in an active pharmaceutical ingredient, Valsartan (an Angiotensin-II-receptor antagonist). Finally, the FDA issued a guidance for the industry, “Control of Nitrosamine Impurities in Human Drugs”, in the fall of 2020. However, the guidelines continue … Continue reading Nitrosamines – An Update »
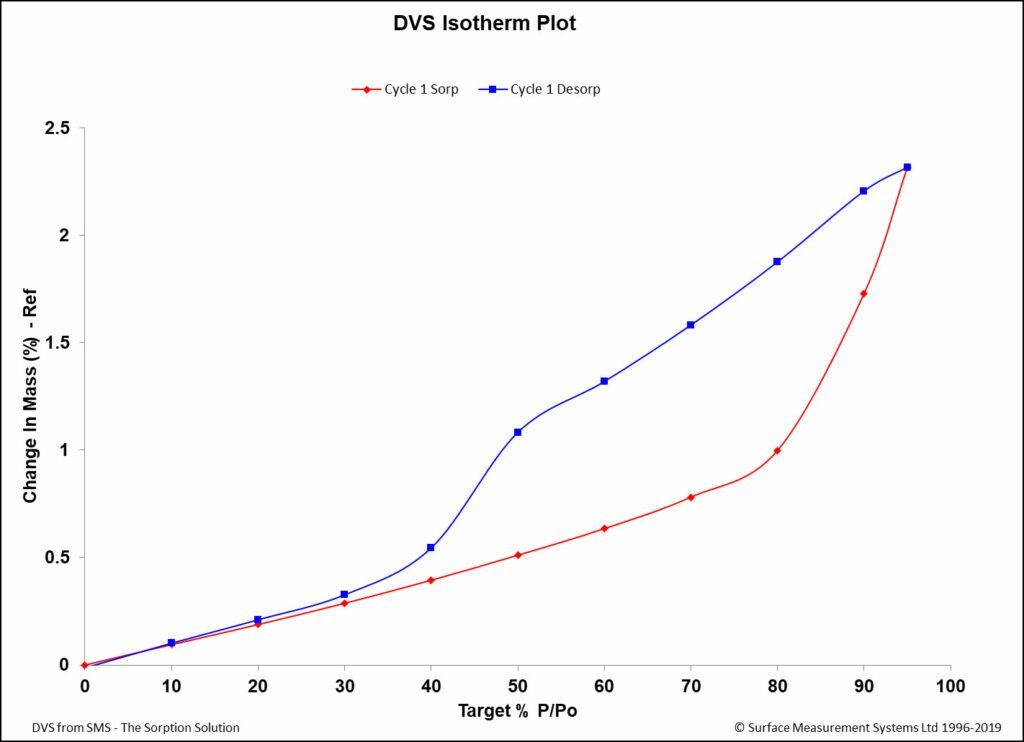
Dynamic Vapor Sorption
Dynamic Vapor Sorption (DVS) is a gravimetric technique used to measure the change in mass of a material in response to changes to surrounding conditions such as temperature or humidity. DVS is primarily used with water vapor but can be applied to other organic solvents as well for the physicochemical characterization of solids. DVS was … Continue reading Dynamic Vapor Sorption »
Keys To Effective Method Development
Effective method development is crucial for the quality control of Active Pharmaceutical Ingredients (API) and Drug Products (DP). Thorough method development enables successful downstream method validation. The regulatory guidance specifies that: Method development and validation vary by application (quantitative, qualitative, etc.). It is phase appropriate. The client may provide additional guidance/validation criteria. The validation guidance … Continue reading Keys To Effective Method Development »
Phase-Appropriate Method Development
The Cost of Drug Discovery and Development and How to Mitigate It The path to successful drug discovery and development is extremely long, expensive, and risky and can take between 10 to 15 years at an average cost of more than $1–2 billion for every new drug that is approved for clinical use.1,2 In fact, … Continue reading Phase-Appropriate Method Development »
TCB* With Your TTC Needs
“The dose makes the poison” – Paracelsus (c. 1493– 1541), born Theophrastus von Hohenheim The Threshold of Toxicological Concern (TTC) refers to levels of mutagenic impurities expected to pose a negligible carcinogenic risk.1 The US FDA, the EMA (European Medicines Agency), and the European Food Safety Authority (EFSA) all have TTC values and regulations in place … Continue reading TCB* With Your TTC Needs »
Forced Degradation Studies Can Reduce Stress(ors)
Forced Degradation is an important addendum to our previous post on Stability and Storage. Stressors are applied to new APIs and drug products to determine their degradation pathways and products under a variety of environmental conditions, including acid, base, light, heat, and oxidation. Forced degradation studies are also known as stress testing, stress studies, stress … Continue reading Forced Degradation Studies Can Reduce Stress(ors) »
The Background, Advantages of, and Considerations for Radiolabeled Peptides
The Background, Advantages of, and Considerations for Radiolabeled Peptides The use of radiolabeled peptides is a well-established tool in researching and treating many diseases and conditions. Selective receptor-targeting peptides are utilized as agents due to their rapid circulatory and tissue clearance and the high affinity and specificity to their targets. Peptides also have a relatively … Continue reading The Background, Advantages of, and Considerations for Radiolabeled Peptides »
Raw Materials Testing: Trust – and Verify – Your Sources
The CGMP guidance for APIs from the FDA states that raw material specifications should be established and documented. The guide’s key line states, “Quality measures should include a system for testing raw materials, packaging materials, intermediates, and APIs. (19.23)”1 All raw materials used in producing APIs for clinical trials must be evaluated by testing or … Continue reading Raw Materials Testing: Trust – and Verify – Your Sources »
Extractables and Leachables
Extractables and Leachables (E&L) are essential areas of concern for the pharmaceutical and food industries, specifically regarding their packaging, usage components (e.g., medical devices or syringes), and the manufacturing chain. We will examine testing of analysis of them within pharmaceutical applications. The two terms are related but distinct, each with its own analytical requirements. Definitions … Continue reading Extractables and Leachables »
Elemental Impurities
A special thanks to Matt Webberley, Associate Director Analytical Research and Development at our sister company, SK biotek Ireland Limited, for his assistance with the profiles of AAS, ICP-OES, ICP-MS, and XRD. A Definition of These Newsmakers Elemental impurities in food have been in the news recently, with reports of everything from lead being found … Continue reading Elemental Impurities »
Nitrosamines in Food and Beverages
This is the third in a series of entries examining nitrosamines in a range of products. Our first of two previous articles presented an overview of nitrosamines, including a historical look at their implication as a probable carcinogen. In the second entry, we reviewed their presence in active pharmaceutical ingredients (APIs), and how to remove … Continue reading Nitrosamines in Food and Beverages »
Nitrosamines in Active Pharmaceutical Ingredients
This is the second in a series of entries examining nitrosamines in a range of products. Our first article presented an overview of nitrosamines, including a historical look at their implication as probable carcinogens. This entry will review their presence in active pharmaceutical ingredients (APIs) and process mitigation strategies. Nitrosamines are organic compounds found in … Continue reading Nitrosamines in Active Pharmaceutical Ingredients »
An Open Letter From Our CEO, Joerg Ahlgrimm to SK pharmteco Customers
Joerg Ahlgrimm, CEO SK pharmteco SK pharmteco January 30, 2023 An Open Letter From Our CEO, Joerg Ahlgrimm to SK pharmteco Customers SK pharmteco’s mission is to improve patient outcomes and save lives by partnering with our customers to produce and deliver life-changing therapies through manufacturing and technical excellence, a positive and adaptable workforce, and … Continue reading An Open Letter From Our CEO, Joerg Ahlgrimm to SK pharmteco Customers »
Nitrosamines: An Overview
This is the first in a series of entries examining nitrosamines in a range of products. Nitrosamines are organic compounds found in the human diet and other environmental outlets. Being potent carcinogens that can cause tumors in nearly all organs, they have been classified as genotoxic impurities (GTIs). There are guidelines and rulings by … Continue reading Nitrosamines: An Overview »
Together AMPAC Fine Chemicals and SK biotek Offer an Expanded Value Proposition in the Worldwide CDMO Space
https://ampacanalytical.com/wp-content/uploads/2018/11/AMPAC_SK-Fine-Chemicals.pdf
AMPAC Fine Chemicals Expands Its Analytical Services to Meet Accelerating Business Demands
https://ampacanalytical.com/wp-content/uploads/2018/05/AA-Expansion.pdf