An Introduction to Dissolution Testing and Development

Dissolution testing is the monitoring of drug substances in a controlled environment from a solid dosage form (i.e., capsules, tablets) to a solution state. These “tests to characterize the dissolution behavior of the dosage form, …also take disintegration characteristics into consideration, are usually conducted using methods and apparatus that have been standardized virtually worldwide over the past decade or so, as part of the ongoing effort to harmonize pharmaceutical manufacturing and quality control on a global basis.”1
Devising a Strategy
When devising a dissolution testing strategy, “a simple but broadly applicable analytical method is always desired.”2 Dissolution analysis is generally performed via UHPLC for faster sample analysis due to the number of samples required. Creating an analytical method should incorporate guidelines from The European Medicines Agency’s (EMA) International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which are also interchangeable with the FDA. These are designed to “avoid redundant testing by (the) industry.”3
It’s the Media…
Dissolution testing is designed to mimic conditions found in the human stomach. As these conditions vary widely from patient to patient, so too should the testing environment. The ranges for the media should allow for various pH levels. These sets should be notated and designed to simulate FaSSGF (fasted state simulated gastric fluid), FeSSIF (fed state simulated intestinal fluid), and FaSSIF (fasted state simulated intestinal fluid). Compendial media is generally HCl or sometimes acetate or phosphate pharmacopeial buffers. As mentioned, with this many simulated environments, along with multiple time sets/points, there will be many samples necessary, and analysis by UHPLC is optimal for timely turnarounds.
We Can Assist with your Dissolution Testing and Development Needs
AMPAC Analytical has years of experience and numerous experts in dissolution testing. We can support release testing, stability, method development, and assist with formulation development through dissolution analysis. We utilize paddle and basket apparatuses in the dissolution phase of testing. Additionally, our equipment offerings include Agilent Infinity II and Thermo Fisher Vanquish Horizon UHPLCs. Please contact us with any specific questions or to receive a quote for your drug product dissolution testing and development needs.
References
- https://www.academia.edu/download/33056117/Pharmaceutical_Dissolution_Testing.pdf#page=15
- Development and Validation of an HPLC Method for Dissolution and Stability Assay of Liquid-Filled Cyclosporine Capsule Drug Products – PMC (nih.gov)
- Q4B Annex 7 (R2): Dissolution Test General Chapter | FDA
Resources



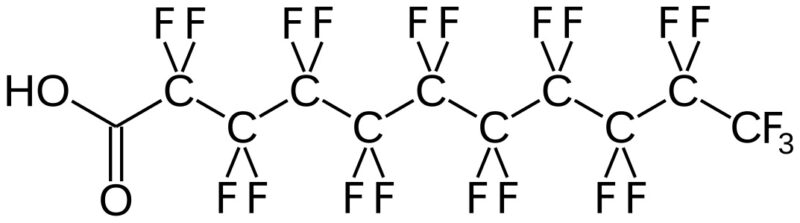
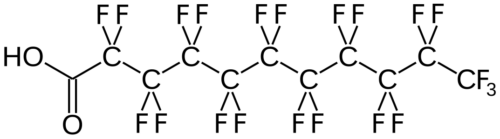 (Per- and) PolyFluoroAlkyl Substances (PFAS) are a class of ubiquitous chemicals that have been found in water, air, fish, and soil across the nation and worldwide. Known as “Forever Chemicals,” there are thousands of different PFAS, and they are present in consumer, commercial, and industrial products.1 Having one of the strongest bonds in organic chemistry, their structures proved to be resistant to heat, water, oil, and degradation.2 They are found in “food packaging and non-stick cookware, cosmetics, waterproof and stain-proof textiles and carpet, aqueous film forming foam (AFFF) to fight Class B fires, and as part of metal plating processes.”3 Teflon and Scotchgard were two of the pioneering products to utilize these fluoropolymers. The two most common PFAS are perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS).
(Per- and) PolyFluoroAlkyl Substances (PFAS) are a class of ubiquitous chemicals that have been found in water, air, fish, and soil across the nation and worldwide. Known as “Forever Chemicals,” there are thousands of different PFAS, and they are present in consumer, commercial, and industrial products.1 Having one of the strongest bonds in organic chemistry, their structures proved to be resistant to heat, water, oil, and degradation.2 They are found in “food packaging and non-stick cookware, cosmetics, waterproof and stain-proof textiles and carpet, aqueous film forming foam (AFFF) to fight Class B fires, and as part of metal plating processes.”3 Teflon and Scotchgard were two of the pioneering products to utilize these fluoropolymers. The two most common PFAS are perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS).