This is the third in a series of entries examining nitrosamines in a range of products. Our first of two previous articles presented an overview of nitrosamines, including a historical look at their implication as a probable carcinogen. In the second entry, we reviewed their presence in active pharmaceutical ingredients (APIs), and how to remove them.
Nitrosamines are organic compounds found in the human diet and other environmental sources. These highly potent carcinogens can cause tumors in nearly all organs and have been classified as genotoxic impurities (GTI).
Background on Nitrosamines in Food and Beverages
The possible linkage between cancer and the large class of chemical compounds known as nitrosamines was first postulated by William Lijinsky in 1970.1 Since then, they have been detected above recommended intake limits in numerous foods and beverages, both naturally occurring and through additives in processed foods. Nitrosamines have been found in a wide variety of different foods ranging from cheeses, soybean oil, canned fruit, meat products, cured or smoked meats, fish and fish products, spices used for meat curing, beer, and other alcoholic beverages.2,3 Beer, meat products, and fish are considered the main sources of exposure. “Drying, kilning, salting, smoking, or curing promotes the formation of nitrosamines.2,4
Nitrites and nitrates may occur naturally in water or foods such as leafy vegetables due to the use of fertilizer or may be added to foods to prevent (the) growth of Clostridium botulinum, or to add color or flavor.”2,5
The nitrosamines most frequently found in food are N-nitrosodimethylamine (NDMA), N-nitrosopyrrolidine (NPYR), N-nitrosopiperidine (NPIP), and N-nitrosothiazolidine (NTHZ).2,3 NDMA, NPYR, and NPIP are reasonably anticipated to be human carcinogens based on evidence of carcinogenicity in animal experiments.2,6,7 Evidence from case-control studies supports an association between nitrosamine intake with gastric cancer, but not esophageal cancer in humans.2,8
Determining Acceptable Levels of Nitrosamine
Levels of nitrosamines have been declining during the past three decades, concurrent with a lowering of the nitrite use in food, use of inhibitors such as ascorbic acid, and application of lower operating temperatures and indirect heating during food processing.2,4

A triple quadrupole MS
The FDA provides “action levels” for poisonous or deleterious substances found in human food and animal feed. These action levels and tolerances represent limits at or above which FDA will take legal action to remove products from the market.9 Current FDA regulations do not limit nitrosamine levels in foods, but they have established an action level of 10 ppb for individual nitrosamines in both consumer and hospital rubber baby bottle nipples. They have also limited the approval of nitrites in curing mixes to the FDA-regulated food additive process (21 CFR 170.60), and the approval of sodium nitrite as a food additive (food preservative) (21 CFR 172.175). The USDA monitors finished meat products to ensure that nitrite is not present in amounts exceeding 200 ppm (9 CFR 424.21).2
As investigators summarized in a study published in the World Journal of Gastroenterology, “there is a positive association between nitrite and nitrosamine intake” and gastric cancer, “between meat and processed meat intake and” gastric cancer and esophageal cancer, “and between preserved fish, vegetable, and smoked food intake and” gastric cancer, “but is not conclusive.”8 While there is not an irrefutable link between nitrite and nitrosamine intake to cancer when combined with action-level requirements and guidance from the FDA, the directive for food and beverage producers is certainly clear.
Final Thoughts
Nitrosamines are an inevitable chemical outcome in the manufacturing and processing of many foods, beverages, medicines, and numerous other products. Due to their low concentrations, they are also challenging to detect. Fortunately, rigorous testing services are available to screen and remove them from exposure by the end user. AMPAC Analytical has the specialized expertise, equipment, and methodologies to detect these impurities by gas chromatography or high-performance liquid chromatography coupled with mass spectrometry. Please contact us with any specific questions or to receive a quote for nitrosamines.
References
Items marked with an asterisk are open access.
- https://doi.org/10.1038/225021a0
- * https://doi.org/10.3390/toxins2092289
- https://ntp.niehs.nih.gov/whatwestudy/assessments/cancer/roc/index.html
- https://onlinelibrary.wiley.com/doi/book/10.1002/9780470430101#page=369
- https://onlinelibrary.wiley.com/doi/book/10.1002/9780470430101#page=566
- * http://ntp.niehs.nih.gov/ntp/roc/eleventh/profiles/s137nsop.pdf
- http://ntp.niehs.nih.gov/ntp/roc/eleventh/profiles/s136nsop.pdf
- * https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4087738/
- * https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-action-levels-poisonous-or-deleterious-substances-human-food-and-animal-feed
Resources & Further Reading
AMPAC
- * Watch Our On-Demand Webinar on Genotoxic Impurities
https://ampacanalytical.com/events/on-demand-webinar/
- * AMPAC Analytical’s Nitrosamine Impurities Testing Webpage
https://ampacanalytical.com/laboratory-services/ampac-analytical-offers-ndma-analyses-of-api-and-dp/#1644962917933-ed2cb310-b026
General Information on Nitrosamines
- * An Organic Chemist’s Guide to N‑Nitrosamines: Their Structure, Reactivity, and Role as Contaminants
https://pubs.acs.org/doi/10.1021/acs.joc.0c02774?ref=pdf - * Report on Carcinogens, Fifteenth Edition: Substances Listed in the Fifteenth Report on Carcinogens
https://ntp.niehs.nih.gov/ntp/roc/content/listed_substances_508.pdf
Nitrosamine and the Diet
- Carcinogenic N-nitrosamines in the Diet: Occurrence, Formation, Mechanisms and Carcinogenic Potential
https://doi.org/10.1016/0165-1218(91)90123-4 - * History of Nitrite and Nitrate in Food (book chapter)
https://doi.org/10.1016/j.chemosphere.2018.02.025 - N-nitrosamines in drinking water and beer: Detection and risk assessment
https://doi.org/10.1007/978-1-60761-616-0_5 - Distribution of Seven N-Nitrosamines in Food
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4609975/



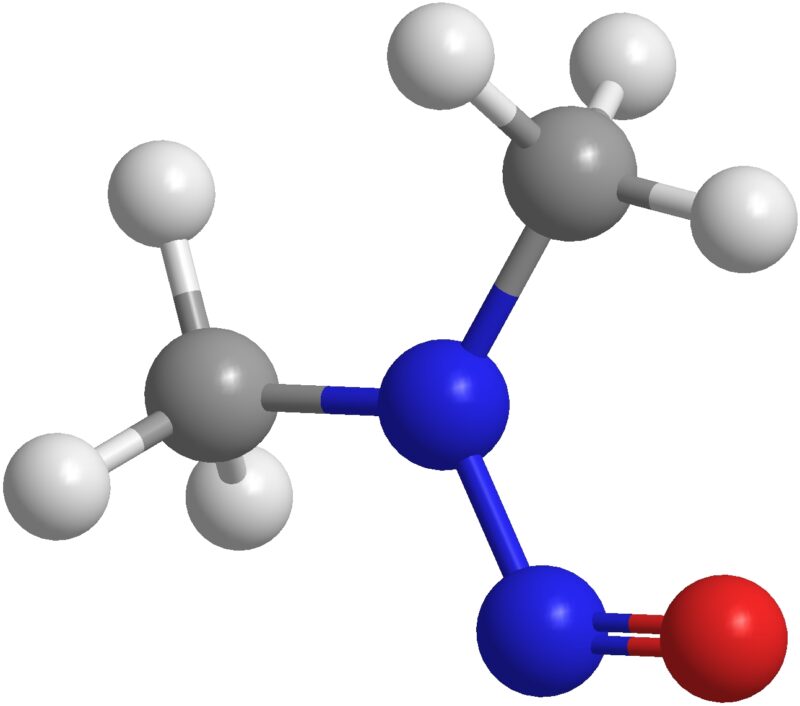
 NDMA
NDMA