The Cost of Drug Discovery and Development and How to Mitigate It
 The path to successful drug discovery and development is extremely long, expensive, and risky and can take between 10 to 15 years at an average cost of more than $1–2 billion for every new drug that is approved for clinical use.1,2 In fact, preclinical drug discovery alone “typically takes five and a half years and accounts for about one-third of the cost of drug development.”3,4 Therefore, even during the earliest stages of a drug product or active pharmaceutical ingredient project, phase-appropriate method development should be instituted to manage costs. This bolsters the chances for success and ensures reliable results, quality management, and reproducibility while avoiding “unreliable results (that) might not only be contested in court but could also lead to unjustified legal consequences for the defendant or to wrong treatment of the patient.”5 At its most basic, phase-appropriate method development maps the “what is needed” to “when it is needed.”6 Effective phase-appropriate method development can provide long-term product support by introducing mass spectrometry compatibility and forced degradation development to ensure your methods are stability-indicating and amenable to unknown impurity identification. By instituting a phase-appropriate method development process, combined with a quality-by-design approach around each logical sequence of events – and rigorously following it – it is more likely to create a cost-effective, successful outcome as you take the drug product through the regulatory process.
The path to successful drug discovery and development is extremely long, expensive, and risky and can take between 10 to 15 years at an average cost of more than $1–2 billion for every new drug that is approved for clinical use.1,2 In fact, preclinical drug discovery alone “typically takes five and a half years and accounts for about one-third of the cost of drug development.”3,4 Therefore, even during the earliest stages of a drug product or active pharmaceutical ingredient project, phase-appropriate method development should be instituted to manage costs. This bolsters the chances for success and ensures reliable results, quality management, and reproducibility while avoiding “unreliable results (that) might not only be contested in court but could also lead to unjustified legal consequences for the defendant or to wrong treatment of the patient.”5 At its most basic, phase-appropriate method development maps the “what is needed” to “when it is needed.”6 Effective phase-appropriate method development can provide long-term product support by introducing mass spectrometry compatibility and forced degradation development to ensure your methods are stability-indicating and amenable to unknown impurity identification. By instituting a phase-appropriate method development process, combined with a quality-by-design approach around each logical sequence of events – and rigorously following it – it is more likely to create a cost-effective, successful outcome as you take the drug product through the regulatory process.
It Can Pay to Outsource
As the incentives for strong phase-appropriate method development increase, so too has the recognition of its value. Unfortunately, “it is not uncommon…to find pharmaceutical companies and contract research organizations (CROs) that are not taking advantage of the phase-appropriate approach and simply reference the typical ICH guidance for analytical items, such as method validation.”7 However, while FDA guidance encourages the use of a phase-appropriate approach, it is lacking in details and requirements. This leaves many companies to seek out ICH guidance as an alternative, conservative approach. Also, within their CGMP quality system, they may find it difficult to accommodate differing levels of CGMP compliance throughout the various clinical phases of development. This is when it might be an opportune moment to consider an outside expert that specializes in phase-appropriate method development processes for drug discovery and validation. A successful yet robust phase-appropriate method development program can balance competing interests and requirements and still provide a regimen that meets the overall development goals without sacrificing any of the requirements of the program.
AMPAC Analytical Laboratories (AAL), an SK pharmteco company, has decades of experience in providing a wide array of release testing services for raw materials, intermediates, APIs, and drug products. Our labs are equipped to handle hazardous, cytotoxic/high potency compounds as well as controlled substances for schedule II through V. Additionally, we have not only the expertise to conduct forced degradation experiments but also appropriate instrumentation like mass spectrometers to support later phases of development for your products. Please contact us to discuss how we can ensure the success of your drug discovery and development project and simultaneously reduce risks.
References
- https://www.sciencedirect.com/science/article/pii/S2211383522000521
- https://www.frontiersin.org/articles/10.3389/fphar.2020.00770/full
- https://www.frontiersin.org/articles/10.3389/fphar.2020.00770/full
- https://www.nature.com/articles/nrd3078
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3658022/
- https://www.pharm-int.com/2020/08/27/phase-appropriate-drug-development-validation-process/
- https://www.pharmtech.com/view/designing-phase-appropriate-cmc-analytical-programs
Resources
- https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q14-analytical-procedure-development-step-2b_en.pdf
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/current-good-manufacturing-practice-phase-1-investigational-drugs
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/inds-phase-2-and-phase-3-studies-chemistry-manufacturing-and-controls-information
- https://www.ich.org/
- www.fda.gov/files/drugs/published/Analytical-Procedures-and-Methods-Validation-for-Drugs-and-Biologics.pdf






 Forced Degradation is an important addendum to our
Forced Degradation is an important addendum to our 





 Elemental impurities in food have been in the news recently, with reports of everything from lead being found in baby food to arsenic, cadmium, and assorted heavy metals in dark chocolate and other foods.
Elemental impurities in food have been in the news recently, with reports of everything from lead being found in baby food to arsenic, cadmium, and assorted heavy metals in dark chocolate and other foods.

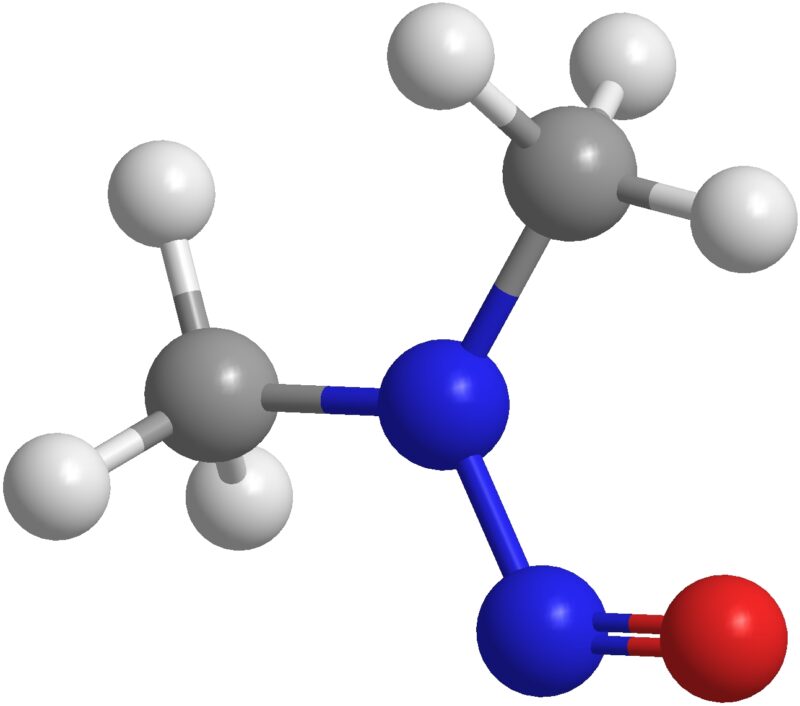
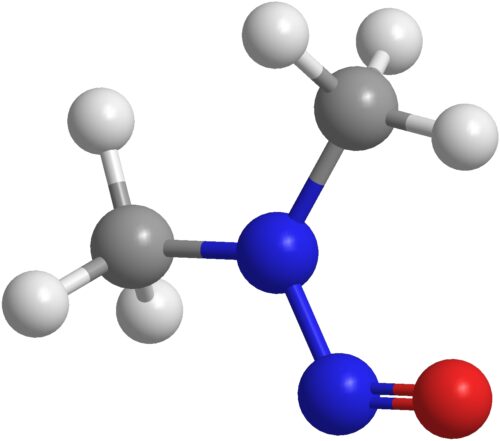 NDMA
NDMA
